Tracers for Groundwater Age Dating
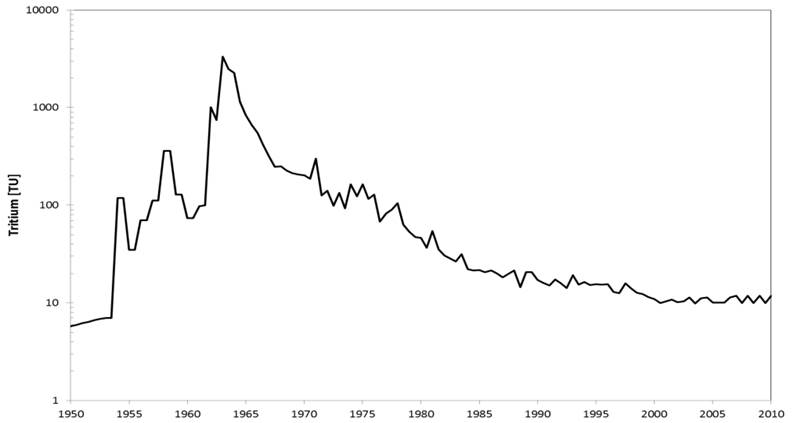
Tritium content in precipitation in Central Europe since 1950 (Source: IAEA, International Atom Energy Agency).
Tritium (3H) is an unstable isotope of hydrogen and has a half-life of 12.4 years. On 1018 hydrogen atoms, 1 tritium atom is accounted for, the total amount of tritium on earth is about 1.8 kg. It is formed in the uppermost layers of the atmosphere by cosmic radiation. The atomic bomb tests of the 1960s brought an anthropogenic tritium input into the precipitation, which was about 1000 times higher than the natural background. Even today, this impulse is still often measurable, which is why 3H is suitable for determining the age of relatively young groundwaters. The natural atmospheric tritium background in precipitation in Central Europe was about 6 TU (1 tritium unit = 0.12 Bq 3H per liter of water or 1 x 3H per 1018 H atoms).
Rule of thumb for qualitative groundwater dating based on tritium content (modified according to Clark & Fritz 1997)
- <0.5 TU water older than 1952 (pre-modern)
- 0.5 – ~ 3 TU mostly mixture of premodern and young (<10 a) water
- 3 – 10 TU mostly younger than 10-15 years or dominant share premodern
- 10 – 20 TU significant proportion of water from the 60s / 70s
- >30 TU water from the 60s / 70s dominate or contamination
- >50 TU additional contamination with tritium
By mixing models (e.g., Piston Flow, Binary Mixing, or Dispersion Model) assuming specific recharge periods, flow paths, and mixture constants, the average residence time of the various age components of a groundwater body can be more accurately calculated.